Water is a transparent, odourless, tasteless chemical compound made of two hydrogen atoms and one oxygen atom. Water is essential for all life forms. It is a universal solvent and regulates temperature.
Water is found as a solid (ice), liquid (water), and gas (water vapour). Water covers about 71 per cent of the Earth's surface. Most of freshwater, about 68.7 per cent, is locked in ice caps. The rest is available through groundwater, lakes, and rivers.
Now what is ice? Ice is primarily the solid, frozen form of water. Ice is formed when the water molecules (two hydrogen, one oxygen) arrange into a crystalline structure when cooled sufficiently. Ice is naturally found on Earth in the form of glaciers, snow, and ice cubes.
So the temperature at which water turns to ice is the freezing point, whereas the temperature at which ice turns to liquid water is called the melting point. But what are these? Let's explore in this article.
What are the freezing and melting points of ice?
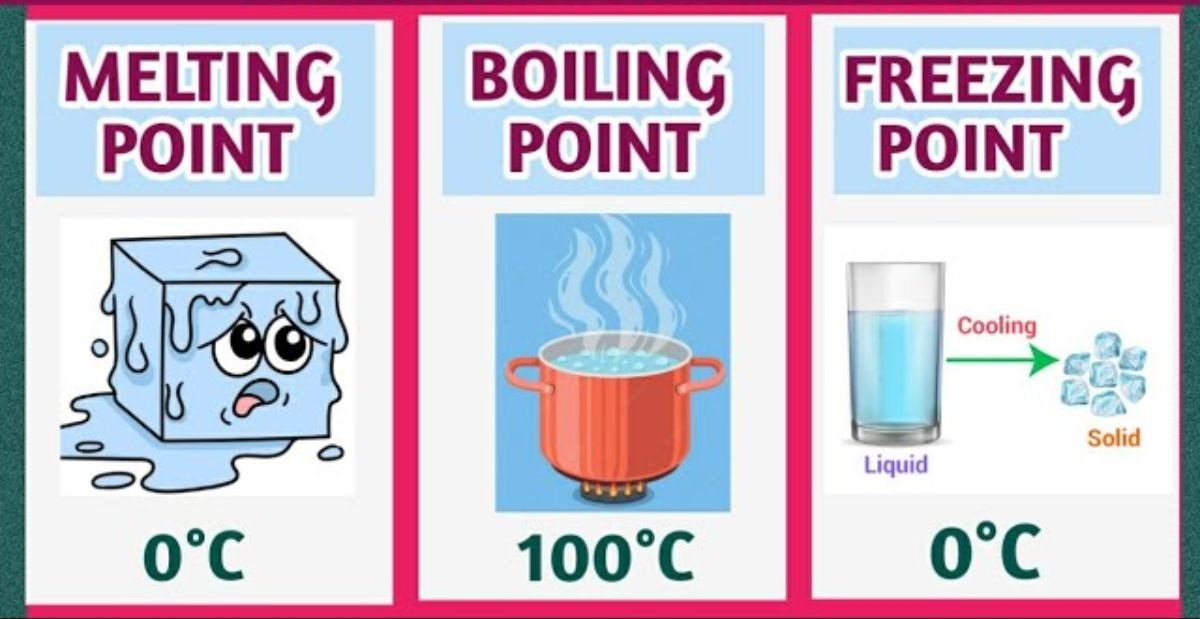
The freezing point (liquid water to ice) and melting point (ice to liquid water) for pure water are the same. That is 0 degress celsius (or 32 degrees farenheit) at standard atmospheric pressure.
Hence, the freezing point is when liquid water turns into solid ice which is 0 degrees celcius.
The melting point is when solid ice turns into liquid water is also at 0 degrees celsius.
Now, factors like impurities (salt) or pressure can slightly alter these freezing or melting points, but 0 degrees celsius.
Science Behind It: Why Does Ice Float On Water?
What is boiling point?
Boiling point is the temperature at which liquid water form converts to gaseous form (water vapour). The boiling point of water is 100 degrees celsius (or 373K).
Interestingly, at high altitudes, the boiling point of water is below 100 degrees celsius because the atmospheric pressure is lower which means water molecules need less energy (heat) to escape into vapour phase.
Comments
All Comments (0)
Join the conversation